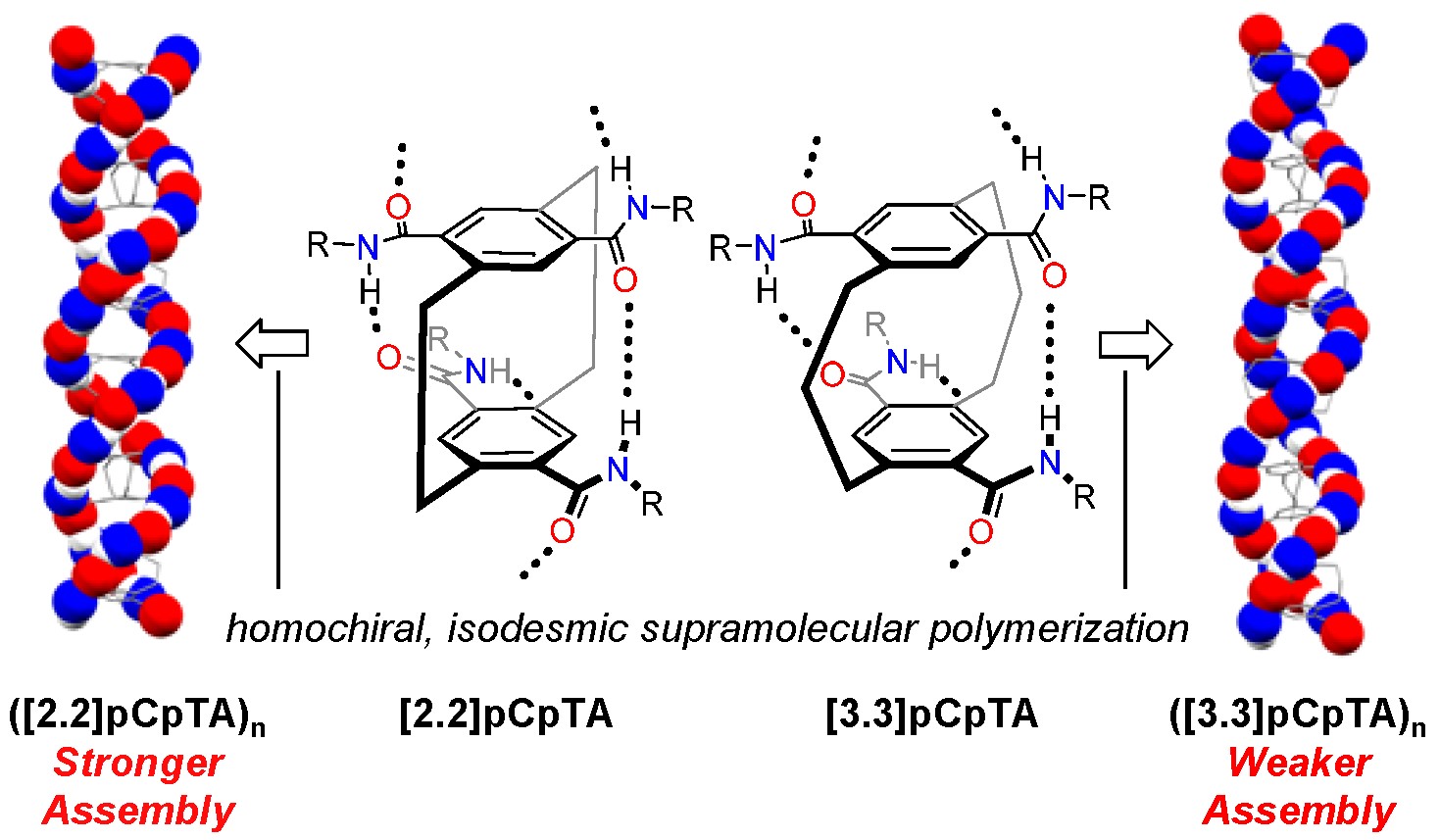
Reported here is the synthesis, characterization, and isodesmic supramolecular polymerization of [3.3]paracyclophane-5,8,14,17-tetracarboxamide ([3.3]pCpTA). The self-assembling monomer, a bridge-expanded homolog of [2.2]paracyclophane-4,7,12,15-tetracarboxamide ([2.2]pCpTA), forms homochiral assemblies in nonpolar solution and the solid state through double helical intermolecular and transannular hydrogen bonding. The additional methylene unit in the [3.3]paracyclophane bridge results in a weakened supramolecular assembly for [3.3]pCpTA compared to [2.2]pCpTA in solution. Likely origins of the change in assembly strength, revealed through X-ray crystallography, computational analysis, and solution-phase spectroscopy, are an increase in (a) the intramolecular and intermolecular deck-to-deck spacing compared to [2.2]paracyclophane resulting from larger amide dihedral angles accompanying transannular hydrogen bonding in the [3.3]paracyclophane, and (b) monomer entropy associated with the scissoring motion of the [3.3]paracyclophane bridge.
