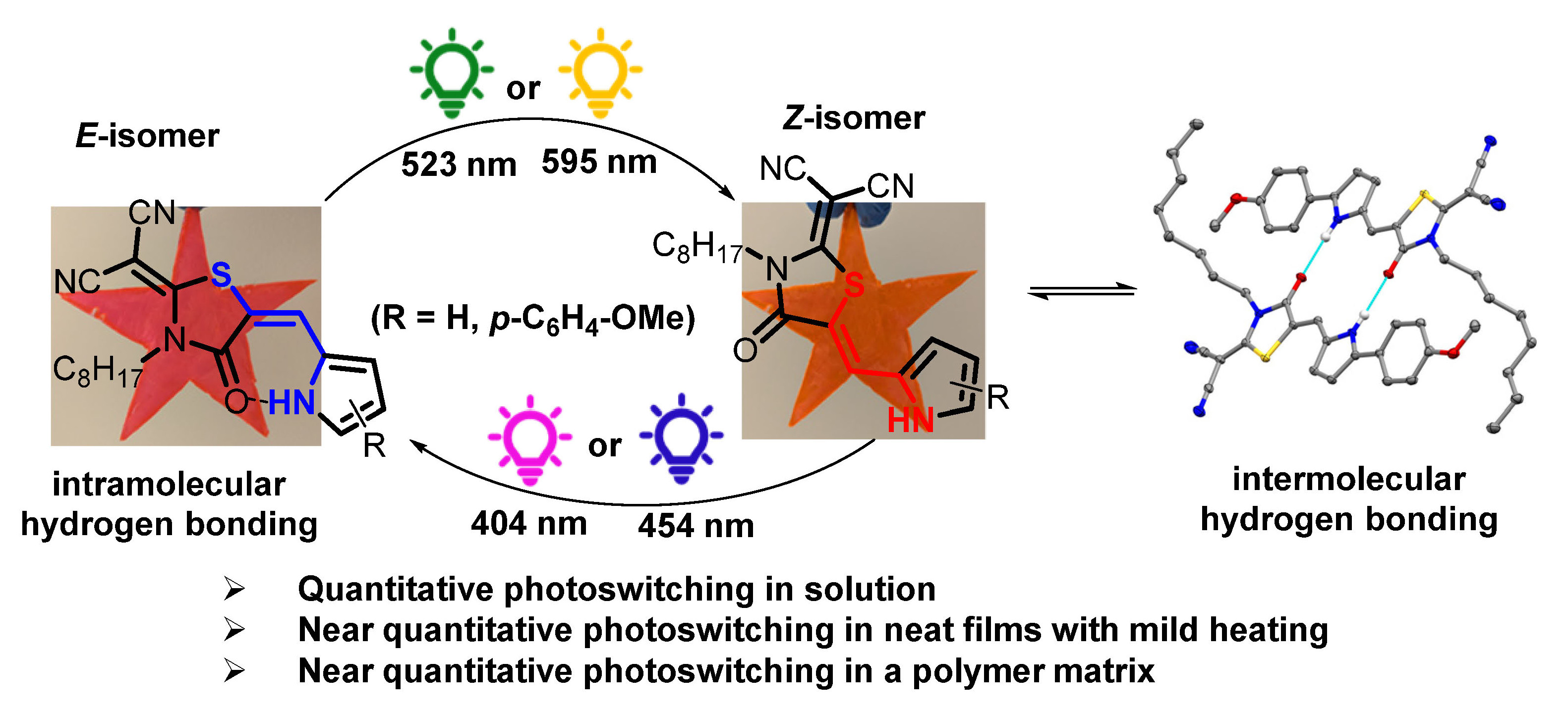
Small molecule photoswitches capable of toggling between two distinct molecular states in response to light are versatile tools to monitor biological processes, control photochemistry, and design smart materials. In this work, six novel dicyanorhodanine-based pyrrole-containing photoswitches are reported. The molecular design avails both the Z and E isomers from synthesis where each can be isolated using chromatographic techniques. Inter- and intramolecular hydrogen bonding (H-bonding) interactions available to the E and Z isomers, respectively, uniquely impart thermal stability to each isomer over long time periods. Photoisomerization could be assessed by solution NMR and UV-vis spectroscopic techniques along with complementary ground- and excited-state computational studies which show good agreement. Quantitative E→Z isomerization occurs upon 523 nm irradiation of the parent compound (where R = H) in solution, whereas Z→E isomerization using 404 nm irradiation offers a photostationary state (PSS) ratio of 84/16 (E/Z). Extending the π-conjugation of the pyrrole unit (where R = p-C6H4-OMe) pushes the maximum absorption to the yellow-orange region of the visible spectrum and allows bidirectional quantitative isomerization with 404 nm and 595 nm excitation. Comparator molecules have been prepared to report how the presence or absence of H-bonding affects the photoswitching behavior. Finally, studies of the photoswitches in neat films and photo-inactive polymer matrices reveals distinctive structural and optical properties of the Z and E isomers, and ultimately afford reversible photoswitching to spectrally unique PSSs using visible light sources including the sun.
