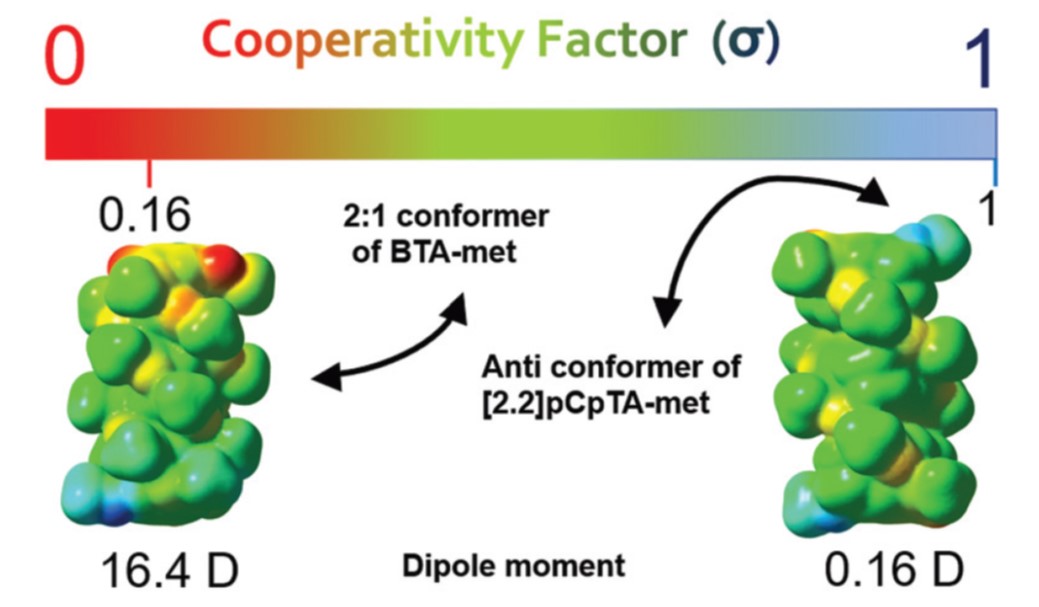
The mechanism by which monomers in solution, beyond a certain concentration or below a certain temperature, self-assemble to form one dimensional supramolecular polymers determines much of the bulk properties of the polymer. The two distinctive pathways of assembly, those of isodesmic and cooperative, can be experimentally identified using spectroscopy and in simulations via a determination of the dependence of the association constant on the oligomer size. Employing large scale free energy calculations, we have been able to show the independence of the free energy change of oligomerization on size in the self-assembly of a [2.2]paracyclophane-tetracarboxamide ([2.2]pCpTA) derivative ([2.2]pCpTA-hex), which is experimentally shown to follow the isodesmic pathway. In contrast, simulations show the free energy change in the case of benzene-1,3,5-tricarboxamide (BTA) to depend on the oligomer size which is a signature of its cooperative nature of self-assembly. These observations are rationalized through the development of a macrodipole moment in BTA oligomers and lack thereof in the [2.2]pCpTA system.
