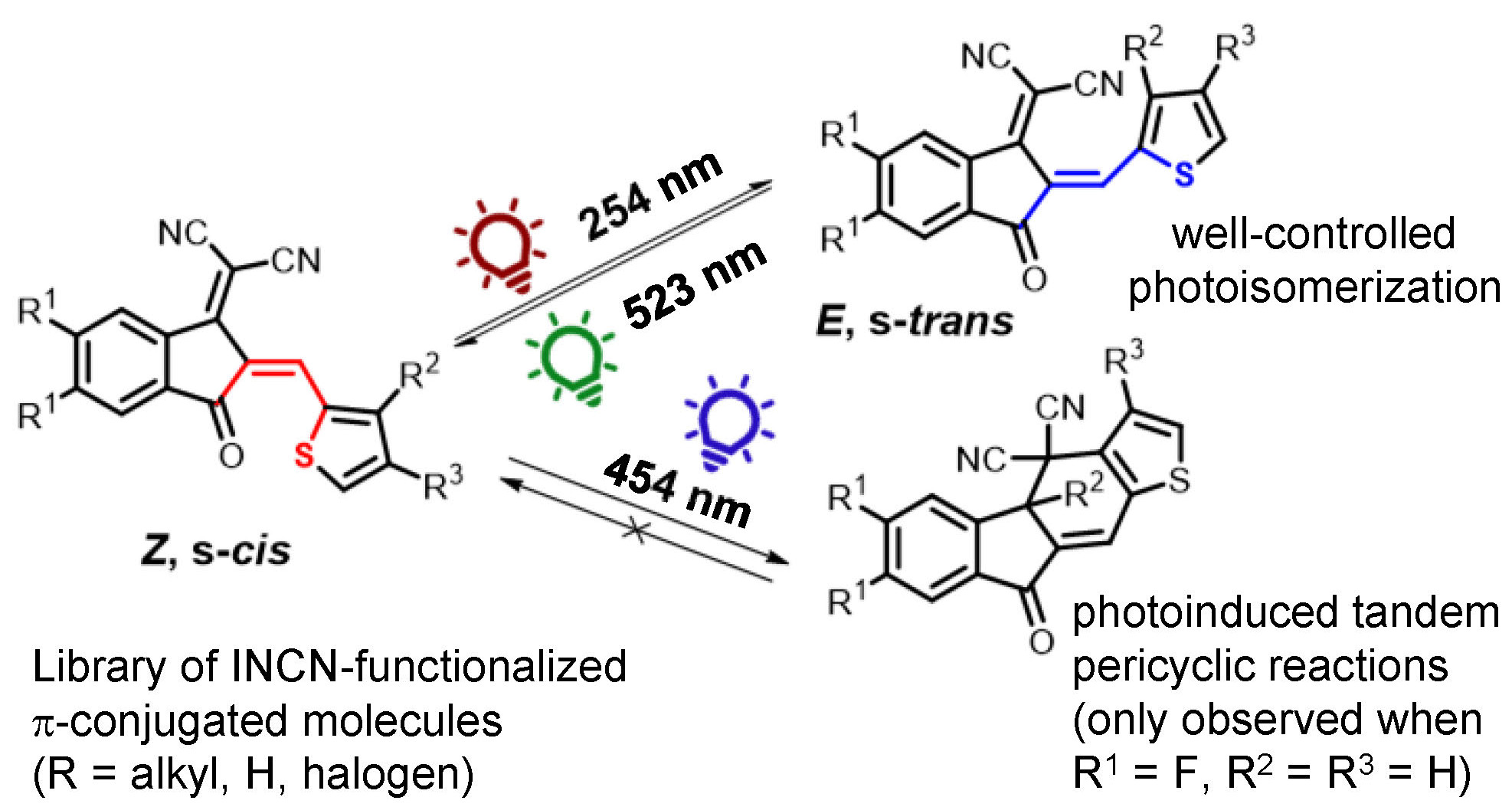
1,1-Dicyanomethylene-3-indanone (INCN) is a popular electron acceptor showcased in hundreds of push-pull oligomers, including some of the best non-fullerene acceptor (NFA) materials used in small molecule-based bulk-heterojunction (BHJ) organic photovoltaics (OPVs). Consequences of the configuration (i.e., Z or E) and conformation (i.e., s-cis or s-trans) of the exocyclic olefin which conjugates INCN to π-conjugated molecules have largely been ignored. Two recent reports have implicated Z/E photoisomerization in the photodegradation of popular NFAs like IT-4F when subjected to broad spectrum irradiation. Here we elucidate through experiments and complementary ground- and excited-state computations the photochemical behavior of a family of eight INCN-functionalized donor-acceptor molecules varying in aryl and heteroaryl substitution, alkyl group substitution, and halogen functionalization on the INCN unit. Well-controlled Z/E photoisomerization using selective wavelengths of excitation spanning the ultraviolet and visible regions is observed in all cases yielding a range of Z/E photostationary state (PSS) distributions with no evidence of a previously reported photooxidation. Z/E photoisomerization followed by sequential pericyclic reactions, consistent with one recent literature report, is identified for just one target molecule upon irradiation at 454 nm. The alkyl group positioning on the thiophene ring neighboring INCN is found to bias the conformational preferences of the target molecules and modulate access to this reaction pathway. All eight molecules undergo facile Z/E photoswitching over numerous cycles upon selective excitation. Overall, the work reveals the well-controlled photochemical behavior of INCN-functionalized π-systems and encourages their usage in the design of future functional organic materials and photoswitches.
