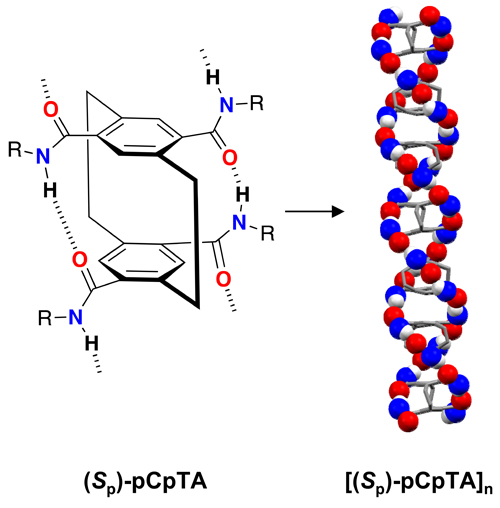
[2.2]Paracyclophane (pCp), unlike many π-building blocks, has been virtually unexplored in supramolecular constructs. Reported here is the synthesis and characterization of the first pCp derivatives capable of programmed self-assembly into extended cofacial π-stacks in solution and the solid state. The design employs transannular (intramolecular) hydrogen bonds (H-bonds), hitherto unstudied in pCps, between pseudo-ortho-positioned amides of a pCp-4,7,12,15-tetracarboxamide (pCpTA) to preorganize the molecules for intermolecular H-bonding with π-stacked neighbors. X-ray crystallography confirms the formation of homochiral, one-dimensional pCpTA stacks helically laced with two H-bond strands. The chiral sense is dictated by the planar chirality (Rp or Sp) of the pCpTA monomers. A combination of NMR, IR, and UV/Vis studies confirms the formation of the first supramolecular pCp polymers in solution.
