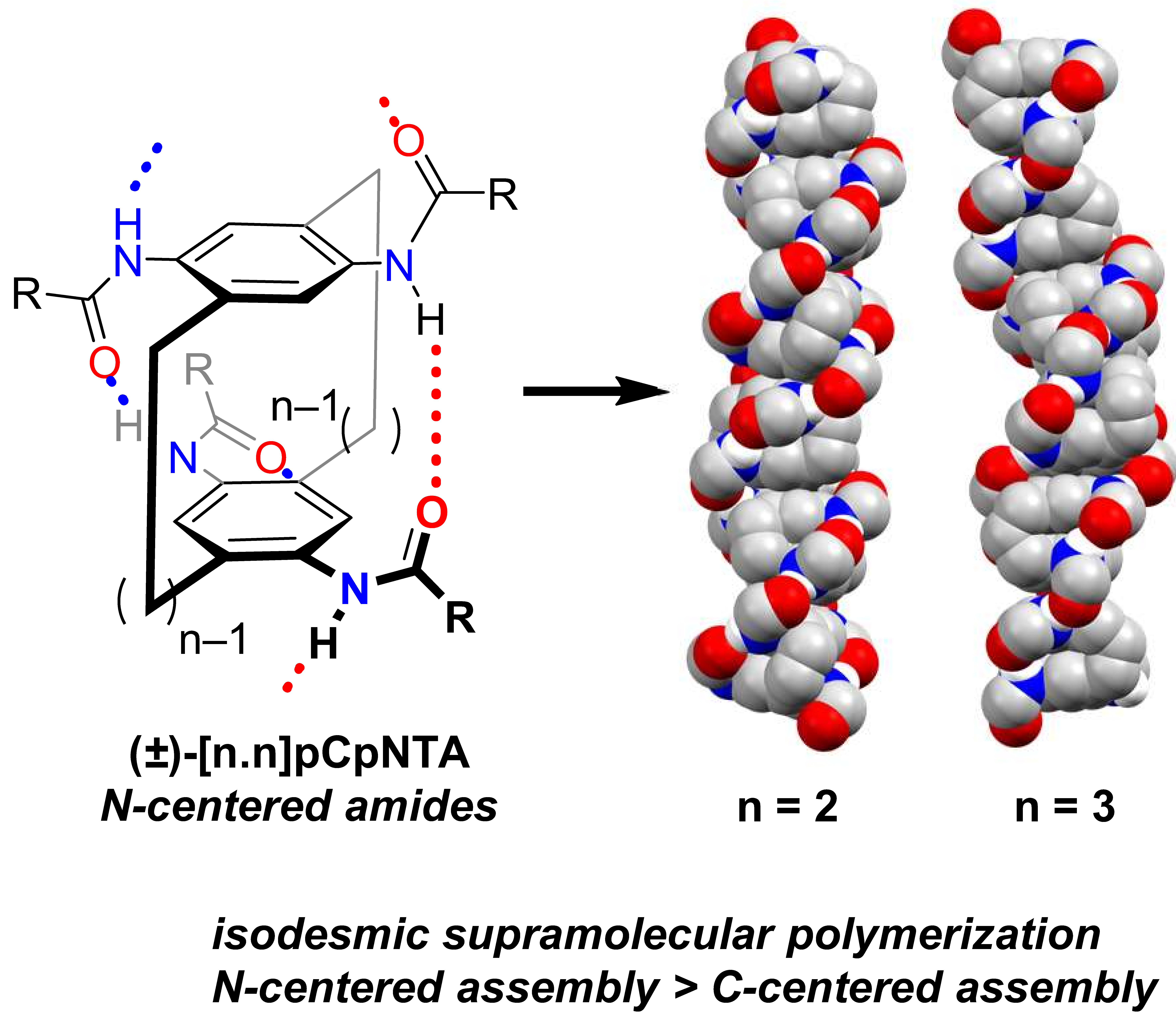
Reported here is the synthesis and self-assembly characterization of [n.n]paracyclophanes ([n.n]pCps, n = 2, 3) equipped with anilide hydrogen bonding units. These molecules differ from previous self-assembling [n.n]paracyclophanes ([n.n]pCps) in the connectivity of their amide hydrogen bonding units (C-centered/carboxamide vs. N-centered/anilide). This subtle change results in a ~30-fold increase in the elongation constant for the [2.2]pCp-4,7,12,15-tetraanilide ([2.2]pCpNTA) compared to previously reported [2.2]pCp-4,7,12,15-tetracarboxamide ([2.2]pCpTA), and a ~300-fold increase in the elongation constant for the [3.3]pCp-5,8,14,17-tetraanilide ([3.3]pCpNTA) compared to previously reported [3.3]pCp-5,8,14,17-tetracarboxamide ([3.3]pCpTA). The [n.n]pCpNTA monomers also represent the reversal of a previously reported trend in solution-phase assembly strength when comparing [2.2]pCpTA and [3.3]pCpTA monomers. The origins of the assembly differences are geometric changes in the association between [n.n]pCpNTA monomers—revealed by computations and X-ray crystallography—resulting in a more favorable slipped stacking of the intermolecular π-surfaces ([n.n]pCpNTA vs. [n.n]pCpTA), and a more complementary H-bonding geometry ([3.3]pCpNTA vs. [2.2]pCpNTA).
