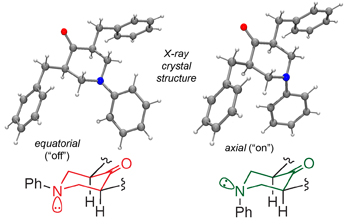
3,5-Disubstituted piperidones provide an opportunity to explore donor–acceptor through-bond interactions in the context of molecular and supramolecular structure. The crystal structure of cis-3,5-dibenzyl-1-phenylpiperidin-4-one 3 is disordered and the lattice accommodates a ~ 3 : 1 ratio of the N-Ph equatorial ( 3–eq) and N-Ph axial ( 3–ax) epimers, based on refined values of occupancy factors. The fortuitous result allows a side-by-side comparison of the two configurations with respect to their donor–acceptor through-bond interactions. The energy difference between 3-ax and 3-eq (DEax–eq) has been evaluated in the gas phase using extensive first principles calculations, and for many levels of theory this difference parallels the experimentally-observed configurational ratio in the solid state (where the epimers share nearly identical packing environments). The calculations further show a difference in the through-bond stabilization for 3–ax and 3–eq, with larger contributions for 3–ax. Natural bond order (NBO) analysis quantifies the delocalization of the donor nitrogen lone pair into the adjacent carbon–carbon bonds and carbonyl acceptor for the 3–ax epimer. The work concludes that molecular-level structural perturbations that arise from or otherwise influence through-bond donor–acceptor interactions have consequences on solid-state and supramolecular assembly structure.
