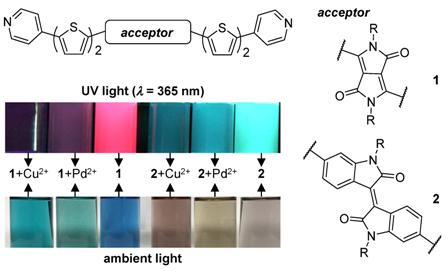
Reported here is the design and synthesis of among the first pyridine terminated acceptor-donor-acceptor-donor-acceptor (A-D-A-D-A) based π-conjugated oligomers, EH_DPP_2T_Pyr (1), EH_II_2T_Pyr (2), and EH_II_1T_Pyr (3). The molecules incorporate thiophenes as electron donors, isoindigo/diketopyrrolopyrrole as electron acceptors, and are capped with pyridine, a weak electron acceptor, on both ends. All target oligomers show attractive photophysical properties, broad absorption in the visible region (λmax = 636 nm, 575 nm, and 555 nm, for 1, 2, and 3, respectively) and emission which extends to the IR region (emission λmax = 734 nm and 724 for 1 and 2, respectively). Given the pyridine nitrogens, the optoelectronic properties of the compounds can be further tuned by protonation/metalation. All compounds show a bathochromic shift in visible absorption and fluorescence quenching upon addition of trifluoroacetic acid (TFA). Similar phenomena are observed upon addition of metals with a particularly strong response to Cu2+ and Pd2+ as indicated by Stern-Volmer analysis (e.g., for Pd2+; Ksv = 7.2 × 104 M-1 (λ = 673 nm), 8.5 × 104 M-1 (λ = 500 nm), and 1.1 × 105 M-1 (λ = 425 nm) for 1, 2, and 3, respectively). The selective association of the molecules to Cu2+ and Pd2+ is further evidenced by a color change easily observed by eye and under UV light, important for potential use in colorimetric sensing.
