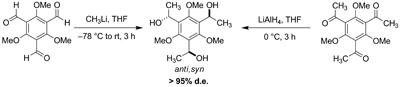
Described are among the first highly diastereoselective, one-pot organometallic addition and hydride reduction reactions (>95% de) involving three symmetry-equivalent carbonyl centers, each that bears a 1,5-relationship to its nearest neighbor. Three-fold methyllithium addition to 2,4,6-trimethoxybenzene-1,3,5-tricarbaldehyde gives the anti,syn triol exclusively (by 1H NMR); addition of HMPA to the reaction or replacement of the substrate’s methoxy groups with ethyl groups affords a statistical 3:1 (anti,syn:syn,syn) diastereomeric product ratio. Analogous asymmetric induction is found upon hydride reduction (using LiAlH4 or NaBH4) of the complementary triketone, 2,4,6-trimethoxybenzene-1,3,5-triethanone. Chelation and steric (gearing) effects about the crowded aromatic core contribute to the observed stereoselectivity.
