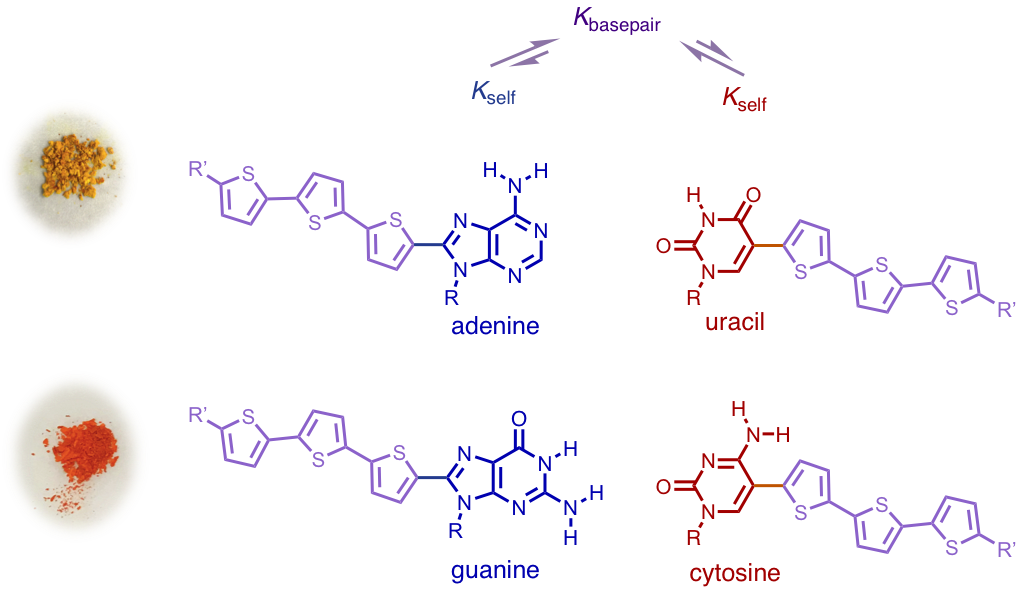
Device-relevant π-conjugated oligothiophenes with the canonical nucleobases directly embedded into the π-framework have been designed, synthesized, and characterized. These oligomers offer the ability to tune optoelectronic properties via the intimate merging of the nucleobase molecular electronic structure with base pairing fidelity. Analysis of their optical and electronic properties in a hydrogen bond disrupting solvent (DMF) indicates that the nucleobase identity influences the intrinsic electronic properties of the semiconductors. These differences are supported by DFT calculations which demonstrate that the HOMO/LUMO orbitals are distributed differently for each compound. The solubility and competition between self-association and base pairing in a hydrogen bond supporting solvent (chloroform) was studied to better understand the oligomer behavior under conditions relevant for downstream solution processing into thin-film devices. These solution studies reveal that in each case base pairing is preferred to self-aggregation; the relatively weak heteroassociation of 1A–1U (35 ± 5 M-1) should be amendable to facile solution processing and successive hydrogen bond formation in the solid-state, while the strong heteroassociation between 1G and 1C (> 104 M-1) should enable assemblies to be preformed in solution. These results are expected to enable the synthesis of more complex π-conjugated architectures and facilitate their extension to optoelectronic devices.
