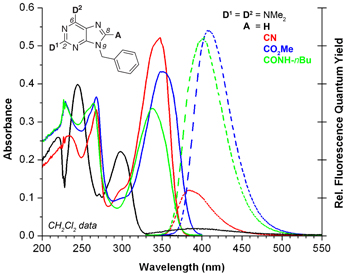
“Push−pull” purines have been synthesized by the introduction of electron-accepting functional groups (A = CN, CO2Me, and CONHR) to the heterocyclic C(8) position to complement typical electron-donating substituents at C(2) (D1) and C(6) (D2). The donor−acceptor purines show significantly altered, and overall improved photophysical properties relative to their acceptor-free precursors (A = H); these include red-shifted (20−50 nm) absorption maxima, highly solvatochromic emission profiles (em lmax from 355−466 nm depending on substitution pattern and solvent) with excellent linear correlations between emission energy and solvent polarity (ET N), improved photochemical stability upon continuous irradiation, and enhanced (up to 2500%) fluorescence quantum yields. Comprehensive structure−property studies show how the absorption/emission maxima and quantum yields depend on donor and acceptor structure, relative donor position (C(2) or C(6)), and solvent (1,4-dioxane, dichloromethane, acetonitrile, methanol, and in some cases water). Further insight regarding electronic structure comes from a quantitative treatment of the solvent-dependent emission data (that provides mge values ranging from 1.9 to 3.4 D) and DFT (B3LYP/6-311++G**) electronic structure calculations. X-ray crystal structures of several derivatives showcase the molecular recognition capabilities of the donor−acceptor chromophores that overall have photophysical and structural properties suitable for applications in biosensing and materials.
