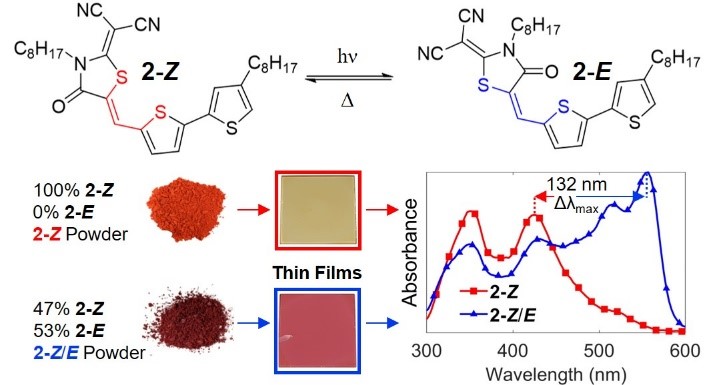
It was recently reported that the most popular electron-accepting units introduced to π-conjugated oligomers studied for organic photovoltaic applications are susceptible to unwanted and even destructive photochemical reactions. Studied here are the consequences of Z/E photoisomerization of the popular 2-(1,1-dicyanomethylene)rhodanine (RCN) unit on the optical and morphological properties of a homologous series of RCN-functionalized oligothiophenes. Oligomers consisting of 1, 2, or 3 thiophene units were studied as pure Z isomers and with E isomer compositions of 25%, 53%, and 45%, respectively, for Z/E mixtures. Solutions of Z isomers and Z/E mixtures were characterized by UV-vis and photoluminescence spectroscopy, wherein changes to optical properties were evaluated based on E isomer content. X-ray diffraction of thin film Z/E mixtures reveals crystalline domains of both Z and E forms after thermal annealing for mono- and bithiophene oligomers, with greater interplanar spacing for E crystalline domains than the Z counterparts along the substrate normal direction. Surface morphology viewed by atomic force microscopy also shows fiber-like structures for the E form with much larger aspect ratio than the Z domains in the bithiophene oligomer. Optical characterization reveals drastic changes in the solid state upon introduction of the E form for the mono- and bithiophene derivatives, whereas subtle consequences are noted for the terthiophene analogue. Most notably, a 132 nm redshift in maximum absorption occurs for the bithiophene oligomer films containing 53% E isomer compared to the pure Z counterpart. Finally, while solid-state photoisomerization experiments find no evidence of Z→E isomerization in polycrystalline Z films, E→Z isomerization is observed and becomes more restrictive in films with higher crystallinity (i.e., after thermal annealing). This structure-property study, which elucidates consequences of RCN configuration on solid-state packing and optical properties, is expected to guide the development of more efficient and stable organic optoelectronics devices.
