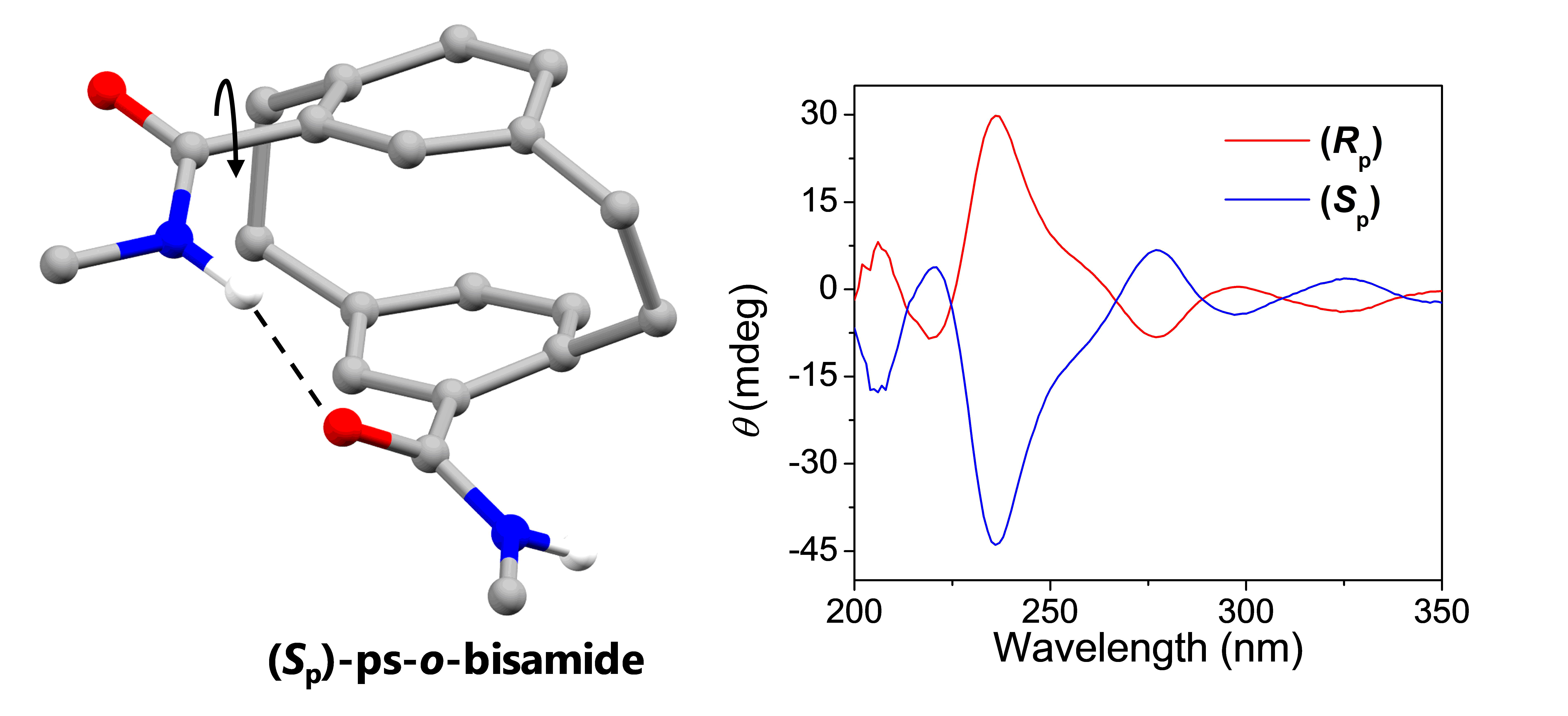
A series of [2.2]paracyclophane‐bisamide regioisomers and alkylated comparators were designed, synthesized, and characterized in order to better understand the transannular hydrogen bonding of [2.2]paracyclophane‐based molecular recognition units. X‐ray crystallography shows transannular hydrogen bonding is maintained in the solid‐state, but no stereospecific self‐recognition is observed. The assignment of both transannularly and intermolecularly hydrogen bonded N–H stretches could be made by infrared spectroscopy, and the effect of transannular hydrogen bonding on amide bond rotation dynamics is observed by 1H NMR in nonpolar solvents. The consequences of transannular hydrogen bonding on the optical properties of [2.2]paracyclophane is observed by comparing alkylated and non‐alkylated pseudo‐ortho 4,12‐[2.2]paracyclophane‐bisamides. Finally, optical resolution of 4‐mono‐[2.2]paracyclophane and pseudo‐ortho 4,12‐[2.2]paracyclophane‐bisamides was achieved through the corresponding sulfinyl diastereomers for circular dichroism studies. Transannular hydrogen bonding in [2.2]paracyclophane‐amides allows preorganization for self‐complementary intermolecular assembly, but is weak enough to allow rapid rotation of the amides even in nonpolar solvents.
