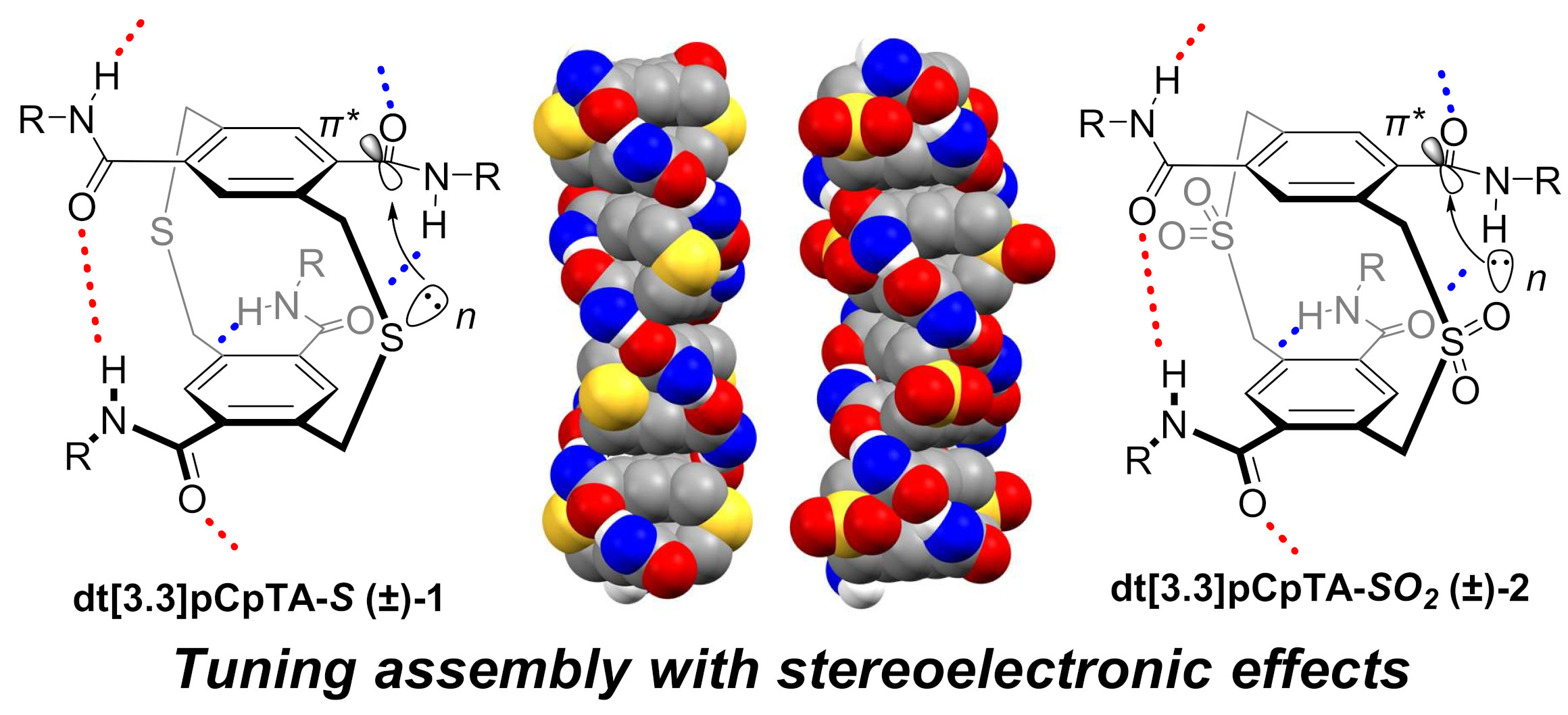
The supramolecular polymerization of 2,11-dithia[3.3]paracyclophanes through self-complementary intermolecular and transannular amide hydrogen bonding is presented. An n→π* interaction between the amide hydrogen bonding units and the central bridging atom results from the single-point exchange of a carbon atom for a sulfur atom. This orbital donor-acceptor interaction can be strengthened by oxidizing the sulfide to a sulfone which acts to shorten the donor⋯acceptor distance and increase orbital overlap. Experimental signatures of the increased n→π* interaction include larger isodesmic polymerization elongation constants in solution, changes in characteristic bond stretching frequencies, and geometric/structural changes evaluated by X-ray crystallography. The experimental data are supported by extensive computational investigations of both assembling and non-assembling 2,11-dithia[3.3]paracyclophanes as well as a rationally designed model system to confirm the role of stereoelectronic effects on supramolecular polymer assembly.
