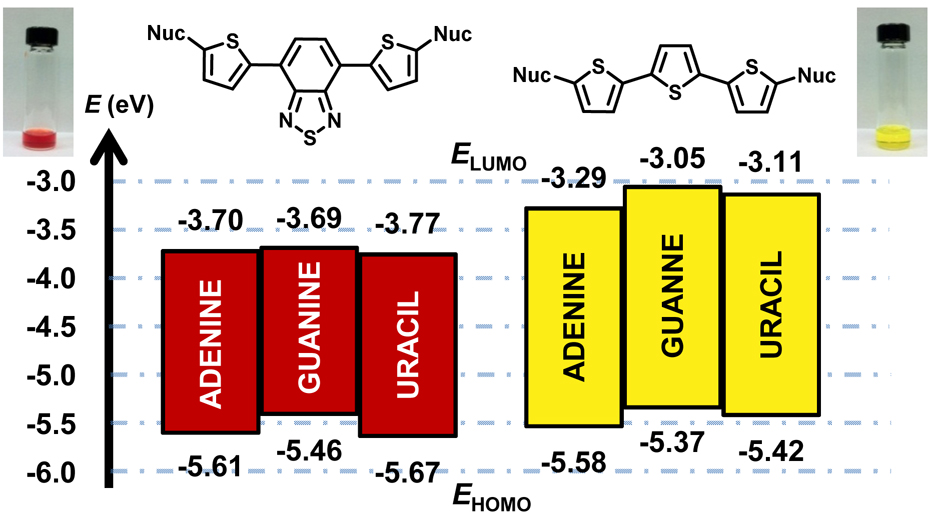
The molecular recognition properties of the nucleobases instruct the formation of complex three-dimensional architectures in natural and synthetic systems; relatively unexplored is their use as building blocks for π-conjugated materials where they might mutually tune electronic and supramolecular structure. Toward this goal, an introductory set (1a–d and 2a–d) of six purine and two pyrimidine-terminated π-conjugated oligomers has been synthesized and used to develop experimental electronic and photophysical structure–property trends. Unlike 2,2′:5′,2”-terthiophene (TTT) derivatives 2a–d, intramolecular charge transfer dominates oligomers 1a–d bearing a 4,7-bisthienylbenzothiadiazole (TBT) spacer due to the strong electron accepting ability of its benzothiadiazole (BTD) ring. The resulting donor-acceptor-donor systems feature lower HOMO–LUMO gaps than the terthiophene-linked nucleobases (ΔEg ~ 1.8 eV vs. 2.4 eV based on electrochemical measurements), and the lowest so far for π-conjugated molecules that include nucleobases within the π-framework. Experiments reveal a dependence of photophysical and electronic structure on the nature of the nucleobase, and are in good agreement with theoretical calculations performed at the B3LYP/6-31+G** level. Overall the results show how nucleobase heterocycles can be installed within π-systems to tune optical and electronic properties. Future work will evaluate the consequences of these information-rich components on supramolecular π-conjugated structure.
