


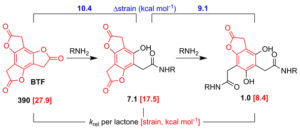
We have made synthetic and mechanistic contributions to the design of molecular scaffolds that allow the rapid and efficient preparation of discrete multifunctional architectures. Seminal work involves benzotrifuranone (BTF), a highly symmetric trilactone capable of one-pot sequential aminolysis to afford a trifunctionalized product. It joins only one other molecular reagent capable of useful single-pot trifunctionalization.
The “weak” inter- and intramolecular interactions that underlie synthetic supramolecular chemistry are also fundamental to enzyme structure, function, and targeting. Over the past several years we have collaborated with researchers in the UF College of Medicine to design and study, using a physical organic approach, novel therapeutics (as antihypertensives and anticancer agents). Some work has shown how small-molecule activation of endogenous Angiotensin Converting Enzyme 2 (ACE2) attenuates, and even reverses, hypertension-linked pathophysiology in vivo. More recent work has introduced Disulfide Bond Disrupting Agents (DDAs) as a unique class of drugs to combat breast cancer tumorigenesis.
The structure- and function-serving roles of the nucleobases are well-appreciated from biological systems. In a different research program we are showing how simple chemical modifications to biorelevant molecules can make them useful for materials applications. We have focused on the nucleobase heterocycles with the idea that their built-in molecular recognition features, showcased otherwise in duplex DNA, can be brought to bear on solid-state architecture, ordering, and even device performance. In our initial work we prepared among the most fluorescent isomorphic nucleobase analogues and subsequently used them in UV-violet OLEDs that provide state-of-the-art performance. We have since focused primarily on introducing the canonical nucleobases (i.e., A, C, G, T/U) to extended (e.g., oligomeric) π-constructs. This work has introduced intrinsically functional and "information rich" heterocycles for organic materials design.
The most sophisticated synthetic and biological assemblies showcase relationships between "weak" inter- and intramolecular interactions and supramolecular function. Understanding and exploiting these relationships as synthetic chemists is critical to bringing new molecular systems to timely and important applications (from analyte detection to novel therapeutics to light harvesting materials). We have been exploring how supramolecular chemistry paradigms borrowed from solution can be used to "program" the arrangement of π-conjugated materials in the solid state to ultimately improve optoelectronic device performance. These studies have recently shown, using a systematic structure-property approach, how hydrogen-bond promoted self-assembly can strengthen π-stacking interactions, improve charge carrier mobility, and lead to favorable optical properties for organic solar cells. Overall, the science is revealing a new "bottom-up" approach to program desirable chromophore structural order in thin film environments. The National Science Foundation is providing funding for this research.