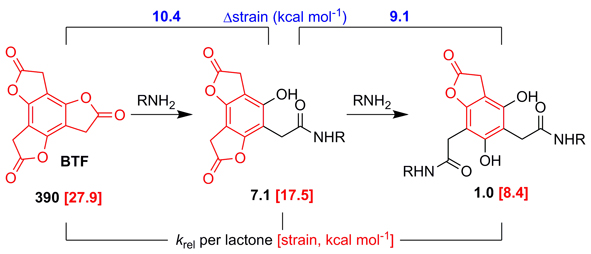
Benzotrifuranone (BTF), bearing three symmetry-equivalent lactone rings, is unique in its ability to undergo highly selective and sequential aminolysis reactions in one-pot to afford multifunctionalized molecules (> 80% overall yield). New insight into this behavior is presented through kinetics measurements (by stopped-flow IR spectroscopy), X-ray crystal structure analysis, quantum chemical calculations, and comparison of BTF to other benzoate esters, including its ring expanded congener benzotripyranone (BTP). While the structure-property investigation confirms stepwise electronic/inductive lactone deactivation for both BTF and BTP, the unusually fast and selective aminolysis of BTF is only fully explained through synergistic ring strain effects. Experimental signatures of the significant ring strain of BTF (~ 28 kcal mol-1 based on DFT calculations vs. 17 kcal mol-1 for BTP) include its high lactone carbonyl stretching energy (1821 cm-1 in acetonitrile vs. 1777 cm-1 for BTP) and bond length alternation within its benzenoid ring. While ring strain is relieved upon the sequential aminolysis of both BTF and BTP, it is only for the former that a ring strain gradient is established that contributes to the stepwise aminolysis rate differences and enhanced selectivity. The work shows how a combination of electronic effects and ring strain can underpin the design of small molecules capable of stepwise functionalization, of which there are notably few examples.
